Prostate cancer diagnosis
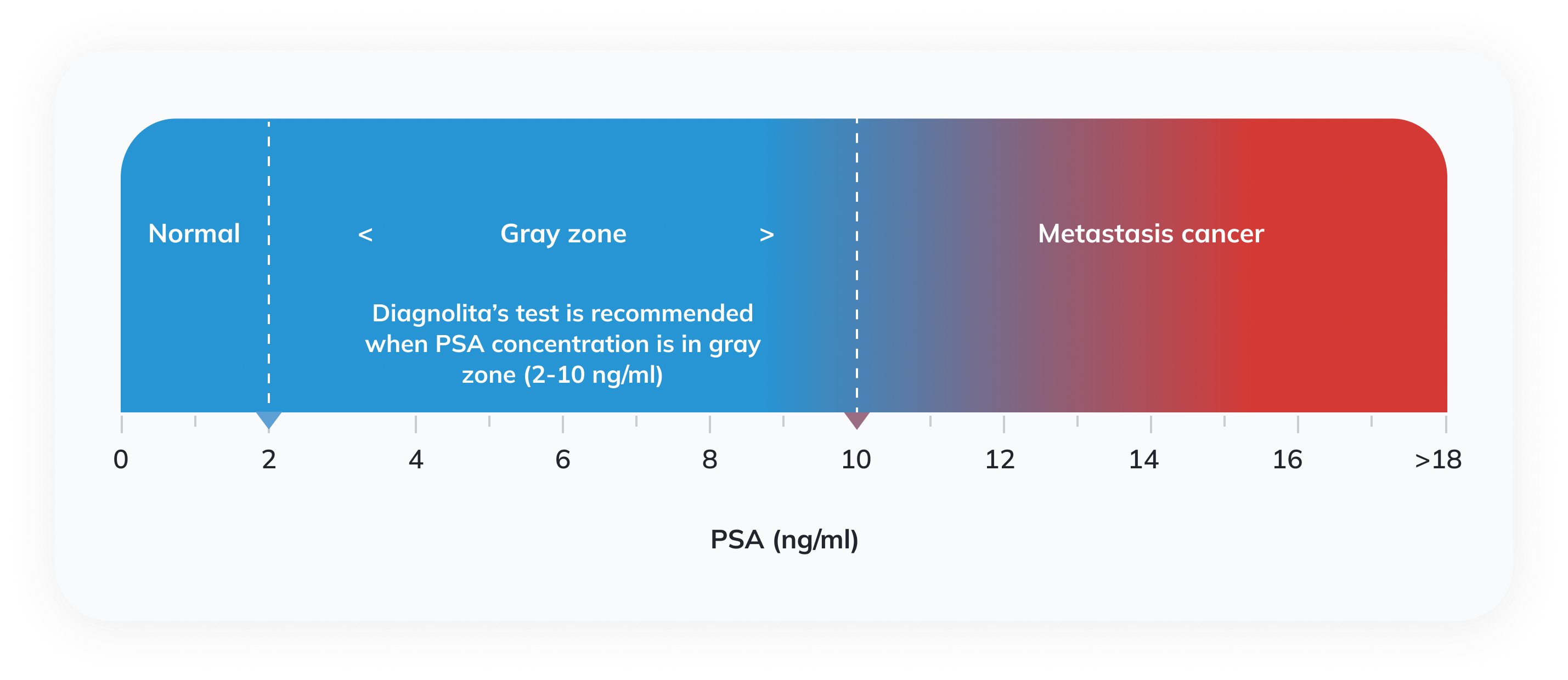
Prostate serum antigen (PSA) is a protein synthesized in the prostate. Some PSA enters the blood, and an increase in PSA levels is often observed in cancer. Currently, the diagnosis of prostate cancer in clinical practice is mostly based on serum PSA levels and digital rectal examination (DRE) by palpating the prostate with a finger through the rectum. Both these tests can show a clear risk of prostate cancer but have significant flaws. Alterations in the prostate are often not palpable, and the increase in PSA concentration is not specific to cancer and only allows one to suspect it. Various prostate diseases cause an increase in PSA levels: benign prostatic hyperplasia, prostatitis, high-grade prostatic intraepithelial neoplasia (HPIN), and prostate cancer. European Urological Association (EUA) recommends using prostate cancer biomarker tests such as the one proposed by Diagnolita when DRE does not show alterations, and the PSA concentration falls into the gray zone (2-10 ng/ml).
Patients with increased blood serum PSA or changes found during DRE usually undergo transrectal prostate ultrasound (TRUS) examination. During this examination, a prostate biopsy may be performed. Examining the biopsy tissue can deny or confirm the diagnosis of prostate cancer. In the case of a positive diagnosis, the histological structure of the cancerous tissue is assessed.
Prostate biopsies after PSA testing and DRE often confirm diagnoses of non-oncological diseases rather than prostate cancer. Another disadvantage of this procedure is the risk of complications caused by an invasive examination. One can reduce the number of redundant biopsies by using prostate cancer diagnostic tests based on the measurement of prostate cancer-specific biomarkers. Diagnolita’s prostate cancer diagnostic test belongs to this type of diagnostics and measures cancer-specific biomarkers in the patient’s urine.
Diagnolita’s biomarker test
Cancer cells in the prostate secrete cancer-specific substances – biomarkers – into body fluids, including urine. Testing urine with sensitive methods can detect cancer biomarkers and assess the risk of prostate cancer. This test is called a liquid biopsy. It is non-invasive, does not cause any complications, and can be repeated many times.
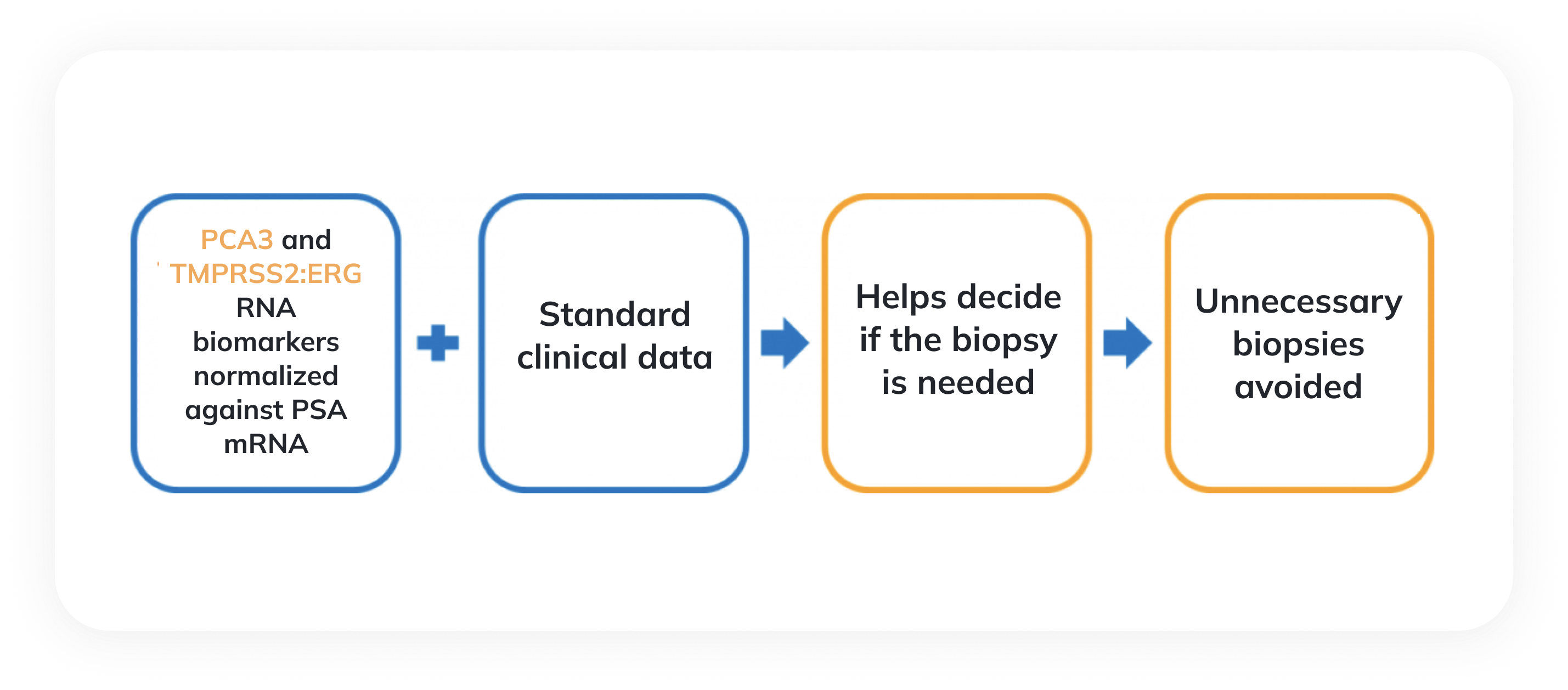
The liquid biopsy test offered by Diagnolita uses a sensitive RT-qPCR method to quantify those biomarkers that, unlike PSA, are specific for prostate cancer. These data, in combination with clinical information (the patient’s age, PSA level, and prostate volume), allow accurate estimation of the risks of prostate cancer and clinically significant prostate cancer. The risk of prostate cancer estimates the probability that the biopsy will be positive. The risk of high-grade cancer evaluates the likelihood that the Gleason score in the biopsy material is 7 points or higher.
In the test results, we provide the threshold values, which help the doctor and the patient to decide whether an invasive biopsy test is necessary. If the calculated risk of significant cancer is below the threshold values the invasive procedure can be avoided or delayed.
If the test results show a high risk of significant cancer, the disease can be diagnosed at an earlier stage and treated effectively.
The quality of Diagnolita’s biomarker test
The prostate cancer diagnostic test is licensed in Lithuania and performed in a modern Diagnolita laboratory in compliance with ISO 15189 requirements. The Diagnolita test has a high specificity compared to the traditionally used blood PSA test. A clinical study carried out in collaboration with Lithuanian clinics and including more than 300 patients proved the efficacy of our test. All these ensure the high quality and reliability of research.
Scientific data. Diagnolita’s non-invasive prostate cancer test.
Quantities of cancer RNA biomarkers are measured in patients’ urine by the RT-qPCR and combined with clinical data to calculate cancer risk. The test predicts the probability of cancer and assesses the risk of significant prostate cancer.
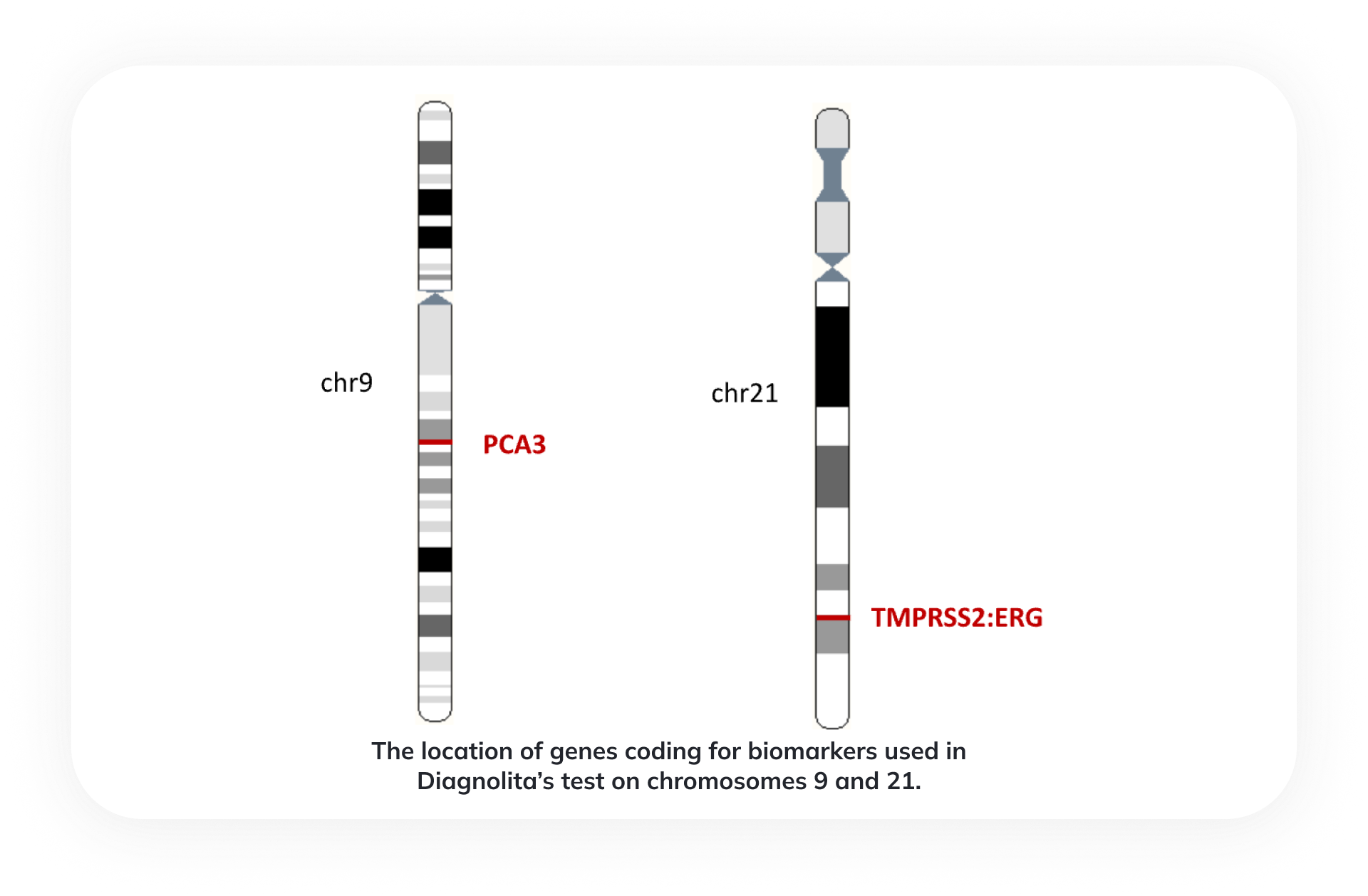
Biomarkers used in Diagnolita’s test
PCA3 (prostate cancer antigen 3) is a non-coding RNA gene specific to prostate tissue. The expression of this gene is elevated in 95% of prostate cancers.
TMPRSS2:ERG (TE) is a gene fusion of the androgen-regulated transmembrane protease gene TMPRSS2 and the transcription factor ERG, which is common in 40-80% of prostate cancers, is also found in HGPIN and is very rarely found in BPH tissues. TE is probably formed in the early stages of prostate cancer development.
Clinical efficacy study results
The clinical effectiveness of the Diagnolita prostate cancer diagnostic test was evaluated after examining a large group of patients in four Lithuanian research centers: National Cancer Institute, LSMU Kaunas clinics, Vilnus University Santaros clinics, and Republican Siauliai hospital.

| Number of patients (PSA 2-10 ng/ml) | Without DRE | After DRE |
| Total number of patients | 295 | 304 |
| Positive biopsy | 146 | 153 |
| Negative biopsy | 149 | 151 |
The comparison of AUC values proves that the accuracy of the Diagnolita test in predicting the risk of significant cancer is much higher than that of the serum PSA test. At the chosen sensitivity of 90%, the specificity of the Diagnolita test is 41%, while the specificity of the PSA test is only 9%.
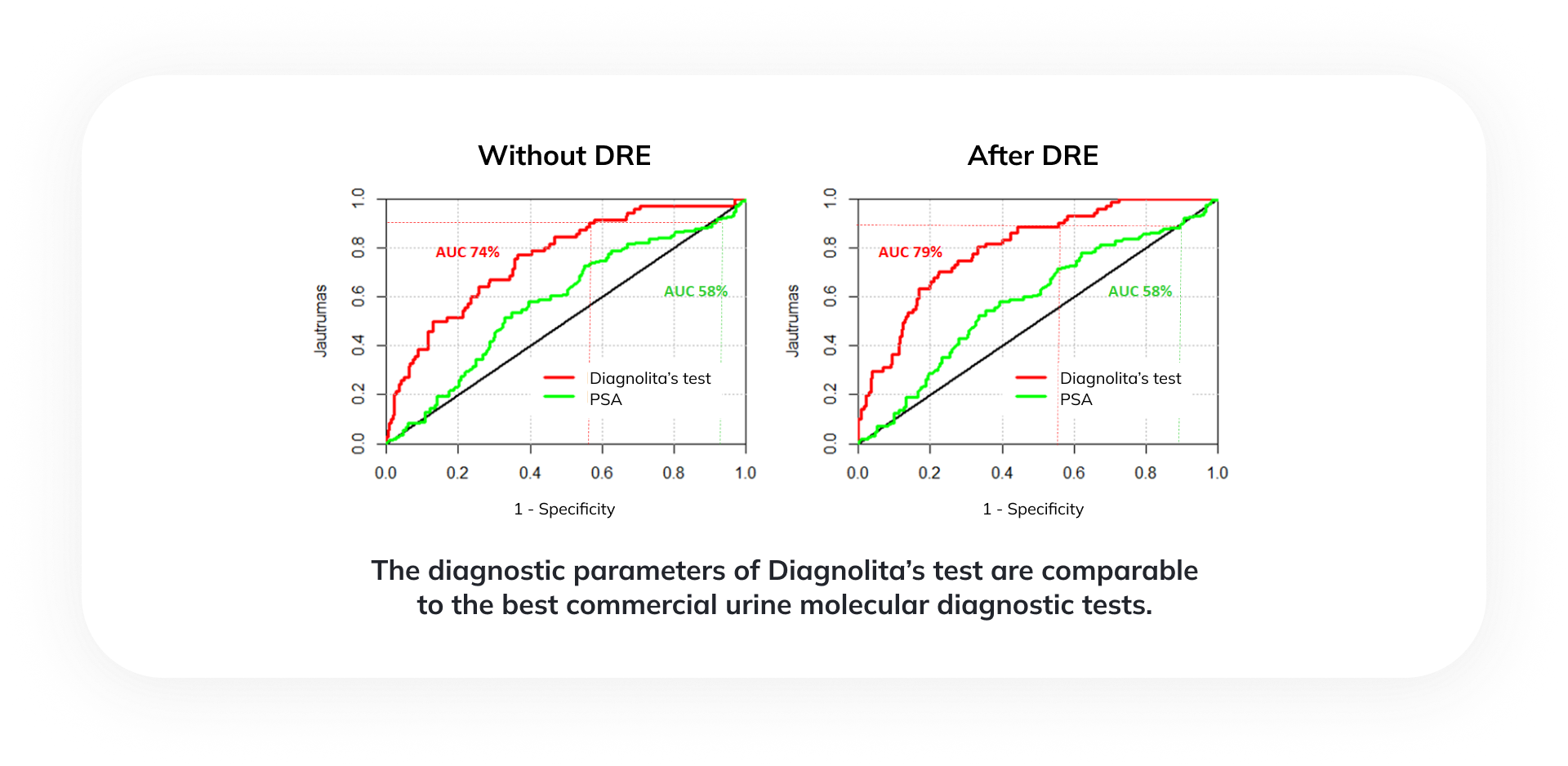
The diagnostic parameters of Diagnolita’s test are comparable to the best commercial urine molecular diagnostic tests. Including only unnecessary biopsies (negative or Gleason 6 cancer) about 40% of such unnecessary procedures could be avoided.
| Prognosis | DRE | AUC | Sensitivity | Specificity | NPV | PPV | Avoided biopsies |
| Prostate cancer (Positive biopsy) | NO | 71% | 92% | 32% | 80% | 57% | 20% |
| YES | 77% | 90% | 40% | 79% | 60% | 25% | |
| Significant prostate cancer (Gleason >= 7, ISUP >= 2) | NO | 74% | 91% | 38% | 93% | 31% | 31% |
| YES | 78% | 89% | 41% | 92% | 32% | 34% |
 Working hours
Working hours 