Moderni neinvazinė diagnostika
Siūlome pasitikrinti sveikatą mūsų specialistų sukurtais tiksliais molekulinės diagnostikos testais. Naudojamos pažangios technologijos šiuolaikiškai įrengtoje laboratorijoje užtikrina tyrimų patikimumą.

Apie Diagnolitą
UAB „Diagnolita“ įkurta 2013 metais. Kuriame molekulinės diagnostikos technologijas ir testus, teikiame medicinos diagnostikos paslaugas. Siūlome modernius neinvazinius tyrimus onkologijos ir infekcinių ligų diagnostikai.
Siekiame, kad žmonės galėtų pasinaudoti naujausiais mokslo laimėjimais savo sveikatos gerinimui.
Šiuolaikiška licencijuota laboratorija
Laikomės darniojo standarto LST EN ISO 15189 ir galiojančių teisės aktų reikalavimų.
Aukštos kvalifikacijos specialistai
Laboratorijoje dirba aukštos kvalifikacijos specialistai, savo srityse sukaupę unikalią darbo patirtį.
Moksliniai ir biomedicininiai tyrimai
9 metų patirtis vykdant mokslinius, biomedicininius tyrimus, naujų diagnostikos metodų kūrimą ir jų validavimą.

Prostatos vėžio diagnostika

Neinvazinės ankstyvosios prostatos vėžio diagnostikos testo paslauga. Testo pagalba, tiriant pacientų šlapimą, tiksliai ir patikimai nustatoma prostatos vėžio tikimybė ir įvertinamas vėžio agresyvumas.

Tai skysčių biopsija paremtas, neinvazinis molekulinis testas, kuris buvo sukurtas mūsų laboratorijoje bendradarbiaujant su žymiausiais Lietuvos urologais ir patvirtintas atlikus mokslinius tyrimus.

Testas leidžia išvengti prostatos biopsijos mažos vėžio rizikos atvejais ir nustatyti didelės rizikos pacientus tolesniam išsamesniam ištyrimui ir gydymui.
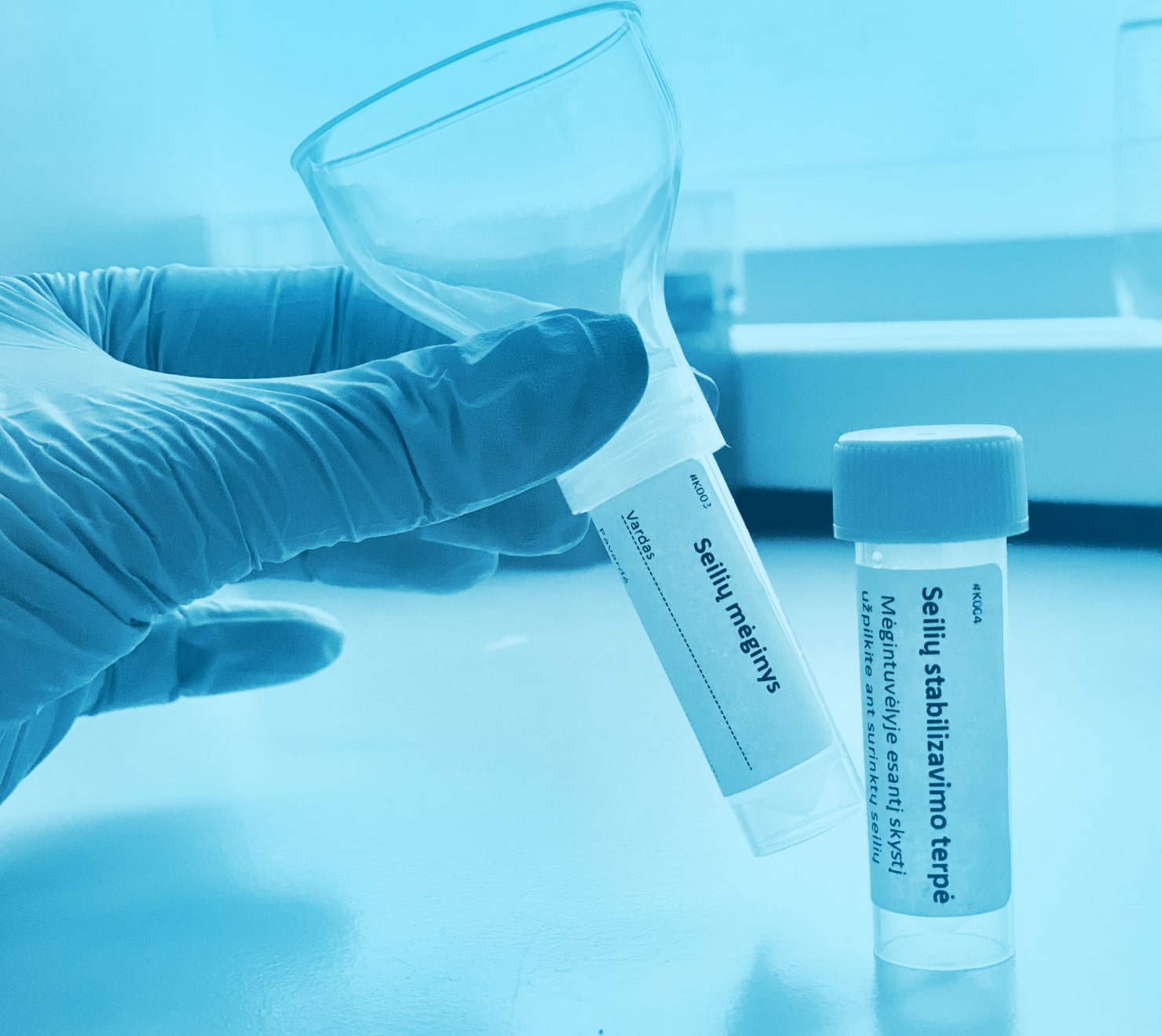
COVID-19 PGR tyrimas iš seilių

Paprastas ir neinvazinis mėginio surinkimas, kurio metu išvengiama nemalonios nosiaryklės mėginio paėmimo procedūros.

Galimybė testą atlikti namuose.

Jautrus ir specifiškas tyrimas – naudojamas PGR metodas.

Sukurtas Lietuvoje UAB „Diagnolita“ laboratorijoje.

Nustatomos visos Lietuvoje esančios SARS-CoV-2 viruso atmainos.
Siūlome bendradarbiavimą įmonėms Vilniuje tikrinant darbuotojus arba klientus. Seilių surinkimo rinkiniai būtų pristatomi į jūsų įmonę, o surinkti mėginiai paimami – nereiktų vykti į specialius punktus. Dėl daugiau informacijos kreipkitės nurodytais kontaktais.

Paveldimo krūties ir kiaušidžių vėžio genetinis tyrimas

Neinvazinis paveldimo krūties ir kiaušidžių vėžio genetinis tyrimas – naudojamas seilių mėginys.

Mėginio surinkimas atliekamas namuose.

Naudojama moderni naujos kartos sekoskaitos technologija.

Naudojami CE-IVD rinkiniai, patikrinti ir verifikuoti UAB „Diagnolita“ laboratorijoje.

Galimybė pasirinkti iš trijų skirtingų apimčių genetinių tyrimų: DiaBOCs BRCA, DiaBOCs HEPA ir DiaBOCs HEPA+.
Turite klausimų?
Susisiekite su mumis nurodytais kontaktais arba užpildykite formą žemiau ir mes Jums atsakysime artimiausiu metu. Taip pat atsakymų į klausimus galite ieškoti mūsų D.U.K. skiltyje.



 Darbo laikas
Darbo laikas 